Advanced Oxidation Technology for any Water Treatment Application
Registered with the EPA as a biocide, PeroxyMAX is formulated to generate large quantities of reactive oxygen species (ROS) for a wide range of uses. Proven in industries as diverse as energy, pulp and paper and water cooling, PeroxyMAX applications include water treatment, microbial control, sulfide removal, well remediation, odor control, cleaning and sanitization, fermentation additive and pulp delignification, bleaching and brightening.
Key PeroxyMAX Features:
ᅠ
- Low/reduced capital and operating costs
- Greater compatibility with other water treatment chemicals
- Less damaging or corrosive to equipment
- Less aggressive towards sensitive metals, polymers and elastomers
- Chemical residuals generally recognized as safe (GRAS FDA designation)
- Ability to target contaminants over other materialsᅠ
- No odor or harmful fumes
- Safer mixing/manufacturing process:ᅠPeroxyMAX components are non-flammable, non-explosive and can be stored on site prior to mixing.
The Chemistry Behind PeroxyMAX
Flexible Deployment Options
The Smart Chemical Treatment Platform
For mobile installations, our PeroxyMAX Oxidation Technology Trailer utilizes smart technology, enhanced automation, data driven quality control and adaptable chemical dosing for improved onsite operations. Quick setup, fast deployment and full cloud-backed connectivity allow PeroxyMAX technology to be quickly applied at any water treatment site.
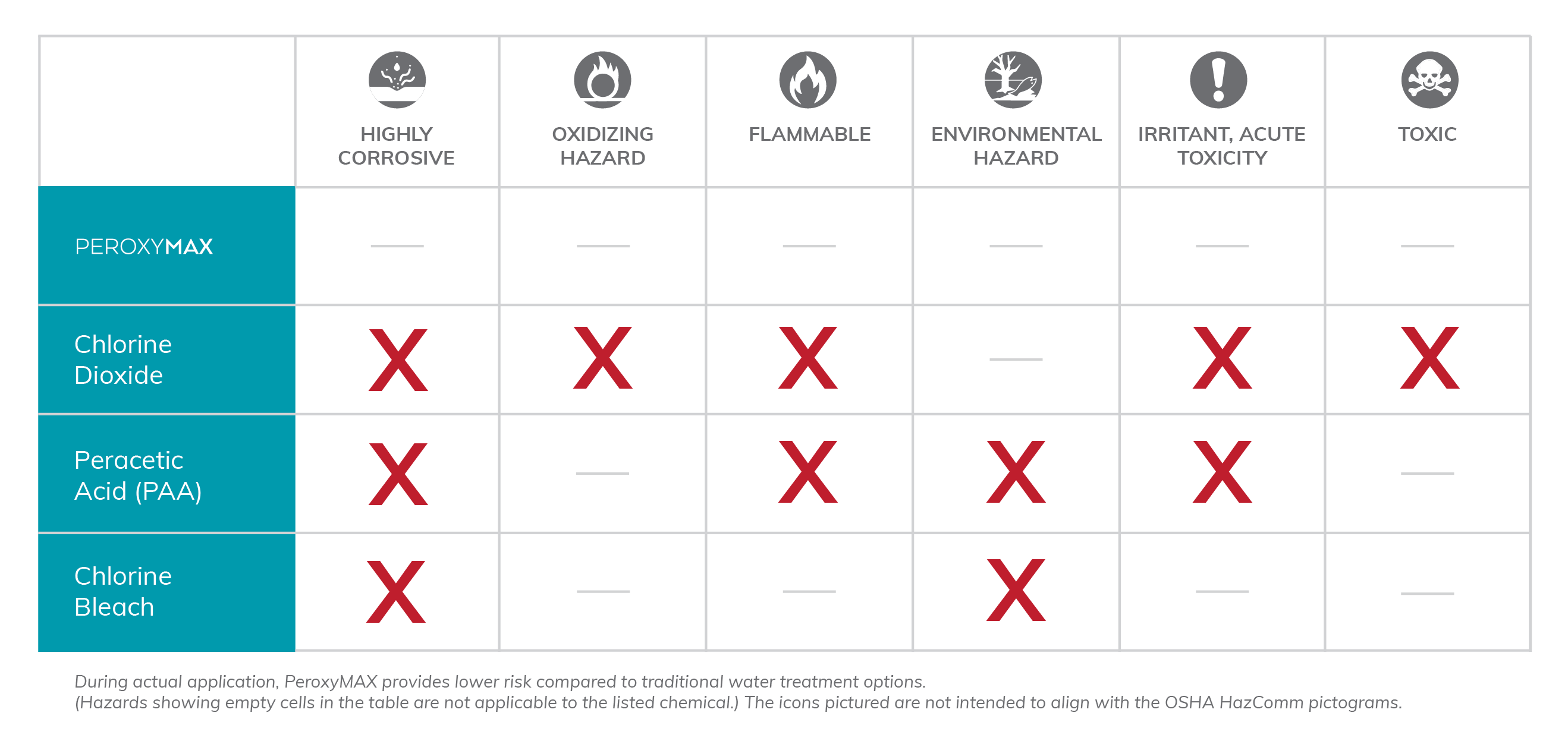
PeroxyMAX™ versus Peracetic Acid
For years, peracetic acid (PAA) has been the go-to oxidizer for water treatment. However, safety and economic concerns have led some industries to look for alternatives. The American Industrial Hygiene Association (AIHA) The Synergist blog lists some of the risks...
Reactive Oxygen Species (ROS): The Secret Behind the Chemistry
Many industries use water as a main component of their operations. With a growing trend toward reuse and recycling, operators from industries as diverse as food processing, paper mills and oil and gas sites are looking at the economics of water treatment. The industry...
Get Started with Clean Chemistry
"*" indicates required fields